Everyone has heard of atoms. We know they exist, I will tell you another time how we became convinced of their existence. What I can say is that it was in the 19th century and that at that time there was no way to be convinced of their existence by seeing them. This gave rise to many scientific and philosophical disputes (do you have to see something to know that it exists?). Nevertheless, much of the tremendous scientific progress of the 20th century was built on the fact that atoms exist…without anyone having ever seen them.
Today, in the 21st century, can we finally see atoms? If we compare an atom to a sphere, it would have the diameter of a tenth of a billionth of a meter, like a hair that you cut a million times, an ångström if you like… But I imagine that this kind of dimension is not very easy to imagine? Let’s say it’s small enough that no optical microscope is powerful enough to distinguish an atom. So, has anyone ever seen an atom?
The answer is yes,
even if “to see” is probably a poor choice of words
To ‘see’ atoms, we can, for example, use a transmission electron microscope (TEM).
The principle of an electron microscope is to use electrons instead of light. This microscope is called “transmission” because the electron beam will pass through the material being observed.
The first electron microscopes were conceived in the 1930s, notably by Ernst Ruska, who was awarded the Nobel Prize in Physics in 1986 “for his fundamental work in electron optics and for the design of the first electron microscope.”
This video (found here) explains how it works

This is a TEM image of a copper (Cu) stack on manganese oxide (MnO).
Each small bright spot is an atom. It looks like the MnO atoms are bigger than the Cu atoms… Except that MnO atoms do not exist, there are Mn atoms and O atoms… and it is not possible to distinguish them on this picture
The atoms are so well arranged periodically that when the electrons pass through this material, they undergo a somewhat unusual path. It is said that they have been diffracted. Finally, thanks to the TEM, what we “see” is not the atoms directly but the way they are organised. Above all, with only this image, it is absolutely impossible to say with certainty which atom it is.
Source : https://t.co/Ee0tPAdEB1
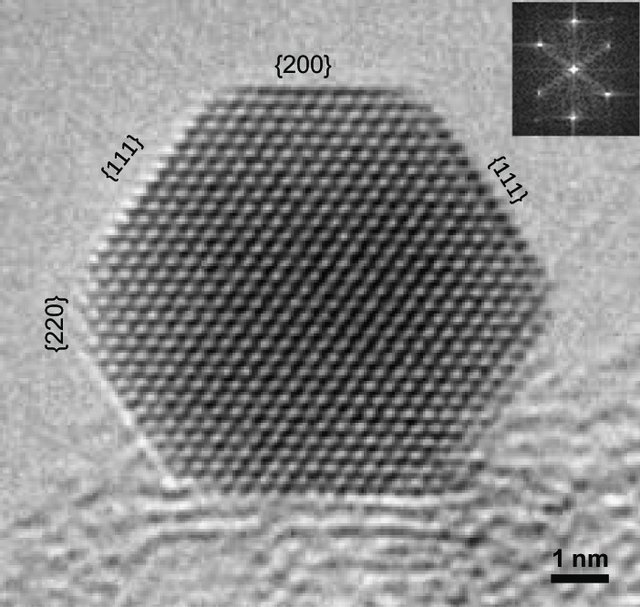
In this picture, the nanoparticle is very structured, with the typical grid pattern of a very good organisation. This organisation is called a crystal. The small numbers between the braces are a notation used in crystallography to identify the orientation of the crystal.
However, underneath the nanoparticle there is a kind of fog. This texture shows that there is matter, but as it is disorganised it is no longer possible to distinguish the atoms that compose it.
source: https://bit.ly/2GR4V25
Another example of a microscope that can “see” atoms is the scanning tunneling microscope (STM). Its principle is to move a tip very close to the surface of the material of interest. The electrical current between the material and the tip is measured, which is a bit special because the tip never touches the surface. The tip is so close (about 1 nanometre) that the electrons have a good probability of passing from the material to the tip! This phenomenon is called the tunnel effect and it depends strongly on the electron supply available in the material.
A short animation on how the STM works is available here
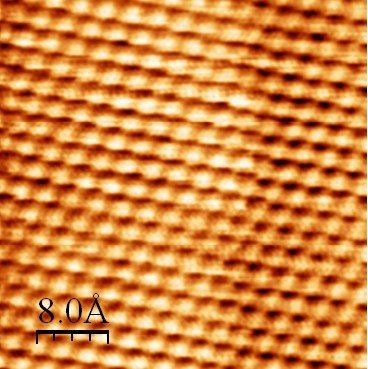
Finally, the STM gives access to the probability of finding an electron at a location. As there are electrons in atoms, it gives an idea of the shape of an atom. For example, here is an image of a gold surface where we can see small organised balls.
This image may look like the TEM images, but it does not give the same information at all.
Source (and other STM images): https://bit.ly/2UnVmQq

One way to see the difference between STM and TEM is to play with the atoms. Here, we can see 48 iron atoms on a copper surface. The interaction of the electrons of each of the atoms causes these kind of circles in the water and this crown of mountains that we call quantum corral.
The density of electrons is greater on the iron atoms, so that they no longer look spherical at all!
Source : https://bit.ly/37Vwgw2
Finally, here is one of the scientific images that I find most extraordinary.
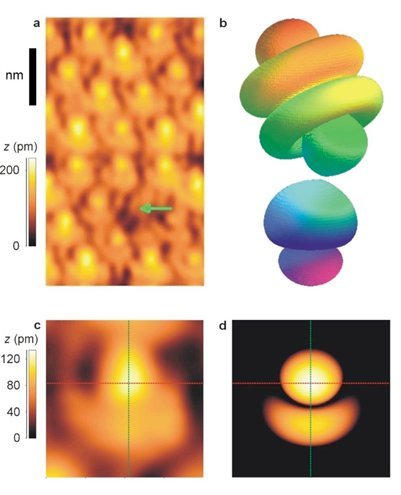
It is still in STM and it has been published a few years ago.
These images are exceptional because we can see strangely shaped atoms. In reality, this is not the case.
We know for decades now that electrons do not revolve around the atomic nucleus as in a planetary system. Electrons are both waves and particles that describe orbits of various shapes. The equations of quantum mechanics can simulate these shapes, but no one had yet obtained experimental proof until then.
Absolutely splendid!
Source : https://bit.ly/2vNsIOf
There are a few other techniques that allow us to “see” atoms, but you can now understandd that the word “see” is not at all appropriate. The training and experience of physicists allow them to see beyond the raw image. This insight is the very first step in the analysis of the results that will allow us to understand a little bit more how matter is formed.